
The Business of Fashion
Agenda-setting intelligence, analysis and advice for the global fashion community.

Agenda-setting intelligence, analysis and advice for the global fashion community.
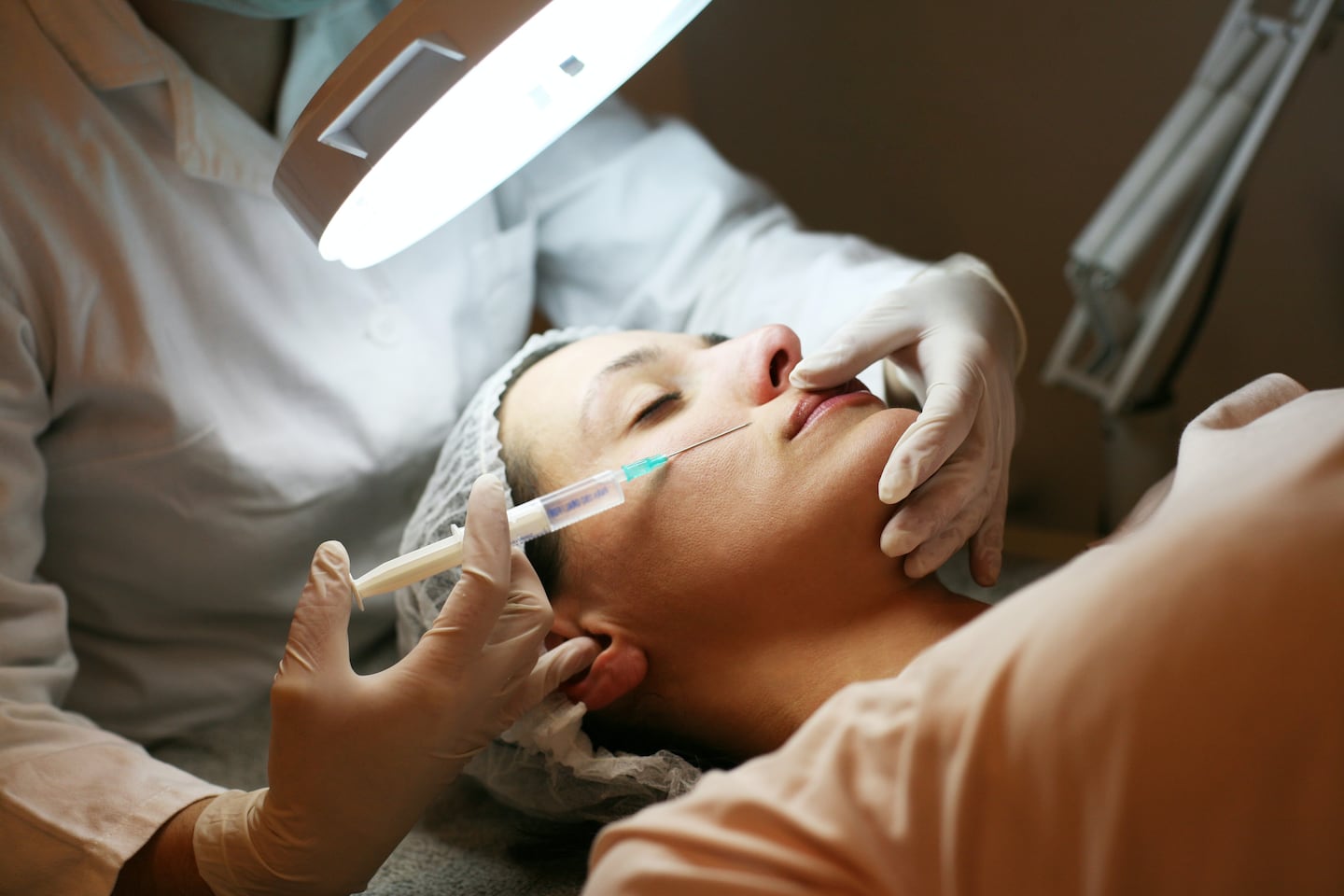
The Centers for Disease Control and Prevention is reportedly investigating several cases of Botox-related illnesses and will warn doctors across the United States about fake Botox injections after several people have been hospitalised.
Health officials in Tennessee and Illinois this week said they were investigating cases of botulism-like illnesses that appeared connected to Botox injections received in a non-medical setting.
There were four patients in Tennessee who reported symptoms that aligned with botulism after receiving cosmetic injections and two were hospitalised, the state health department said in a statement last week. Officials said they believed the injections were from a counterfeit product.
Botulism is caused when a toxin attacks the body’s nerves and causes muscle weakness in the face, mouth and throat. Symptoms can include blurred vision, drooping eyelids, slurred speech and difficulty swallowing, according to the Tennessee department of health. A purified form of the bacteria that causes the condition is used in Botox and approved by the Food and Drug Administration, according to the Illinois department of public health.
ADVERTISEMENT
“Receiving these treatments in unlicensed, unapproved settings can put you or your loved ones at serious risk for health problems. Please only seek cosmetic services under the care of licensed professionals trained to do these procedures and who use FDA-approved products,” said Sameer Vohra, director of the Illinois department of public health. “If you are experiencing any health problems after a recent cosmetic treatment, please contact your healthcare provider immediately for help and assistance.”
Two people in Illinois were also hospitalised and reported botulism-like symptoms after receiving “either Botox or a possibly counterfeit version of the product”, the Illinois department of public health said in a statement. Both patients had received injections from a licensed nurse in LaSalle county who administered the procedure outside of her authority, according to the agency.
By Sam Levine
Learn more:
Cosmetic fillers and Botox are more normalised than ever before — but consumers are also more weary of looking overdone. Skinvive, the first “injectable moisturiser” to receive FDA approval, may be able to fill in the gaps.
Going public is usually a pivotal moment in a company’s history, cementing its heavyweight status and setting it up for expansion. In L’Occitane’s case, delisting might be a bigger conduit for growth.
Brands say they’re barreling ahead with marketing and commerce on the app, even as the clock starts ticking for owner ByteDance to sell it or shut it down.
The Spanish beauty and fashion conglomerate’s smart acquisitions and diverse portfolio could be a big draw for investors. Plus, Adidas is set to confirm its stellar first quarter.
How not to look tired? Make money.